- 0.02 0.084 Variable yield tactical nuclear weapon—mass only 23 kg (51 lb), lightest ever deployed by the United States (same warhead as Special Atomic Demolition Munition and GAR-11 Nuclear Falcon missile). AIR-2 Genie: 1.5 6.3 An unguided air-to-air rocket armed with a W25 nuclear warhead developed to intercept bomber squadrons.
- R= Rydberg Constant 1.0974x10 7 m-1; λ is the wavelength; n is equal to the energy level (initial and final) If we wanted to calculate energy we can adjust R by multipling by h (planks constant) and c (speed of light).
Section 4.1: The Mole Concept and Atoms. Atomic mass unit 1 amu = 1.661 X 10-24 g (this is roughly equal to the mass of ONE proton) Because the mass of one amu is so small, chemists deal with a much larger number of atoms while working with chemicals.
The Hiroshima equivalent has been pegged at exactly 15 kilotons of TNT, 1 which is itself defined as being equivalent to 62.76 terajoules, or 15 Tera calories. Here is an online Earthquake Equivalent to Hiroshima atomic bombs calculator which helps you to compare the earthquake energy to the Hiroshima atomic bombings level. Enter the magnitude of the earthquake and the tool will compare the value and tell you its equivalent energy of Hiroshima atomic bombs.
Earthquake Equivalent To Hiroshima Atomic Bombs
The Hiroshima equivalent has been pegged at exactly 15 kilotons of TNT, 1 which is itself defined as being equivalent to 62.76 terajoules, or 15 Tera calories. Here is an online Earthquake Equivalent to Hiroshima atomic bombs calculator which helps you to compare the earthquake energy to the Hiroshima atomic bombings level. Enter the magnitude of the earthquake and the tool will compare the value and tell you its equivalent energy of Hiroshima atomic bombs.
Formula:
Energy = 10 4.8Energy Released = (10 (1.5 x Magnitude)) x EnergyH = Released Energy / (6.27x1013)Example
An earthquake has been recorded with a magnitude of 8 Ritcher scale. Find the earthquake energy equivalent to Hiroshima Atomic Bombings
Energy =104.8
=63095.734448
Energy Released = 10(1.5 x 8) x 63095.734448
=1012 x 63095.734448
=6.30957x1016
Hiroshima Equivalent = 6.30957x1016/(6.27x1013)
=1006.3116
Related Calculators:
Top Calculators
Popular Calculators
Top Categories
Atomic Mass
Atomic mass is based on a relative scale and the mass of 12C (carbon twelve) is defined as 12 amu.
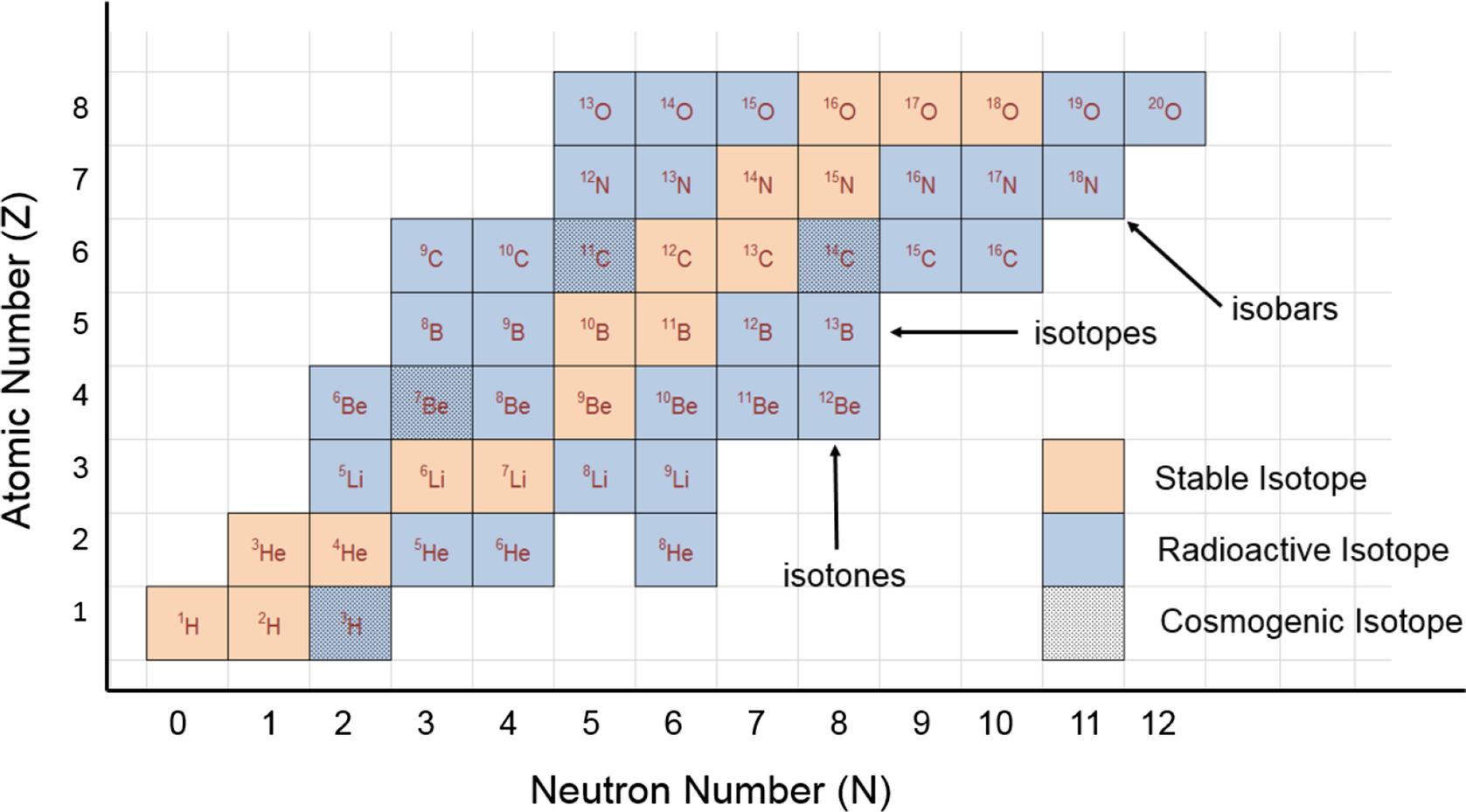
Why do we specify 12C? We do not simply state the the mass of a C atom is 12 amu because elements exist as a variety of isotopes.
Carbon exists as two major isotopes, 12C, and 13C (14C exists and has a half life of 5730 y, 10C and 11C also exist; their half lives are 19.45 min and 20.3 days respectively). Each carbon atom has the same number of protons and electrons, 6. 12C has 6 neutrons, 13C has 7 neutrons, and 14C has 8 neutrons and so on. Since there are a variety of carbon isotopes we must specify which C atom defines the scale.
Deck set 2 0 13 0. All the masses of the elements are determined relative to 12C.
By the way, the mass of an element is not equal to the sum of the masses of the subatomic particles of which the element is made!
Average Atomic Mass
Since many elements have a number of isotopes, and since chemists rarely work with one atom at a time, chemists use average atomic mass.
On the periodic table the mass of carbon is reported as 12.01 amu. This is the average atomic mass of carbon. No single carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is 12.01 amu.
Why 12.01 amu?
Atomic 1 0 4 Equals Grams
If a sample of carbon was placed in amass spectrometer the spectrometer would detect two different C atoms, 12C and 13C.
The natural abundances of 14C, 10C and 11C are so low that most mass spectrometers cannot detect the effect these isotopes have on the average mass. 14C dating is accomplished by measuring the radioactivity of a sample, not by actually counting the number of 14C atoms.
The average mass of a carbon is calculated from the information the mass spectrometer collects.
The mass spectrometer reports that there are two isotopes of carbon,
98.99% of the sample has a mass of 12 amu (not a surprise since this is the atom on which the scale is based).1.11% of the sample has a mass of 13.003355 amu (this isotope is 1.0836129 times as massive as 12C)
The average mass is simply a weighted average.
ave. mass = 12.01 amu
(Yes, the number 12.01 has the right number of significant figures, even though 1.11% only has 3 significant figures.)
If we know the natural abundance (the natural abundance of an isotope of an element is the percent of that isotope as it occurs in a sample on earth) of all the isotopes and the mass of all the isotopes we can find the average atomic mass. The average atomic mass is simply a weighted average of the masses of all the isotopes. Net framework 4.30319 free download.
| Isotope | Atomic Mass amu | Natural Abundance % |
| 16O | 15.99491 | 99.759 |
| 17O | 16.99913 | 0.037 |
| 18O | 17.99916 | 0.204 |
(Yes, the sig figs are correct.)
Another kind of question could be asked.
Copper has two isotopes 63Cu and 65Cu. The atomic mass of copper is 63.54. The atomic masses of 63Cu and 65Cu are 62.9296 and 64.9278 amu respectively; what is the natural abundance of each isotope?
substituting gives
(eq. A)One equation and two unknowns.is there another equation? If there is another equation we would have two equations and two unknowns, and a system of two equations and two unknowns is solvable.
Dirt 4 1 0 179 free download. Since there are only two major isotopes of Cu we know that
or
(eq. B)
Use eq. B to substitute for %63Cu in eq. A.
To the correct number of significant figures
Of course, a question like the one above could be turned around another way.
Gallium, atomic mass 69.72 amu, has two major isotopes, 69Ga, atomic mass 68.9257 amu, and 71Ga. If the natural abundance of each isotope is 60.00 and 40.00 % respectively what is the mass (in amu) of 71Ga.
69.72 amu = (0.6000 x 68.9257 amu) + (0.4000 x 71Ga)71Ga = 70.9249 amuThe mole
| Element | mass of 1 atom (amu) | mass of 100 atoms (amu) |
| H | 1.0079 | 100.79 |
| C | 12.01 | 1,201 |
| W | 183.9 | 18,390 |
What is the relative mass of 1 C atom as compared to 1 H atom?

What is the relative mass of 100 C atoms as compared to 100 H atoms?
C:H = (1,201/100.79):1 = 11.92:1What is the relative mass of 1 W atom as compared to 1 H atom?
W:H = (183.9/1.0079):1 = 182:1What is the relative mass of 100 W atoms as compared to 100 H atoms?
W:H = (183.9/1.0079):1 = 182:1The point here? As long as the number of atoms remains the same the relative mass does not change.
Atoms are small, and it is possible to place 1.0079 g of H on a balance (possible but not easy in the case of hydrogen).
It is also possible to place 183.9 g W, or 12.01 g of C on a balance.
Now, I state with absolute certainty that I have placed the same number of atoms on each balance! How do I know? I know because the relative masses of the samples on the balance, are the same as the relative masses of the individual atoms.
W:H = (183.9 g/1.0079 g):1 = 182:1C:H = (12.01 g/1.0079 g):1 = 11.92:1
The number of atoms I placed on the balance is know as a mole.
What is the relative mass of 100 C atoms as compared to 100 H atoms?
C:H = (1,201/100.79):1 = 11.92:1What is the relative mass of 1 W atom as compared to 1 H atom?
W:H = (183.9/1.0079):1 = 182:1What is the relative mass of 100 W atoms as compared to 100 H atoms?
W:H = (183.9/1.0079):1 = 182:1The point here? As long as the number of atoms remains the same the relative mass does not change.
Atoms are small, and it is possible to place 1.0079 g of H on a balance (possible but not easy in the case of hydrogen).
It is also possible to place 183.9 g W, or 12.01 g of C on a balance.
Now, I state with absolute certainty that I have placed the same number of atoms on each balance! How do I know? I know because the relative masses of the samples on the balance, are the same as the relative masses of the individual atoms.
W:H = (183.9 g/1.0079 g):1 = 182:1C:H = (12.01 g/1.0079 g):1 = 11.92:1
The number of atoms I placed on the balance is know as a mole.
Atomic 1 0 4 Equals Equal
For many years the number of atoms in a mole remained unknown; however, now it is know that a mole of atoms contains 6.02214 x 1023 atoms. Meta trader mac.
So, the periodic table provides us with a great deal of information.
The periodic table lists
the mass of an atom in amu,the mass of a mole of atoms (i.e. the molar mass) in grams,
and the mass of 6.02214 x 1023 atoms in grams